Research Article - International Journal of Pharmaceutical, Chemical and Biological Sciences ( 2022) Volume 12, Issue 4
Antiretroviral Treatment Response among People on First-line Antiretroviral Treatment in Amhara Region, Ethiopia
Minwuyelet Maru Temesgen1*, Daniel Dagne1, Addisu Tesfie1, Asefa Missaye1, Gizachew Yismaw2 and Andargachew Mulu32Department of Microbiology and Parasitology, Amhara Public Health Institute, Ethiopia
3Department of Health Sciences, Armauer Hansen Research Institute, Ethiopia
Minwuyelet Maru Temesgen, Department of Research and Technology transfer, Amhara Public Health Institute, Dessie, Ethiopia,
Received: 01-Aug-2022, Manuscript No. IJPCBS-22-71204; Editor assigned: 03-Aug-2022, Pre QC No. IJPCBS-22-71204 (PQ); Reviewed: 17-Aug-2022, QC No. IJPCBS-22-71204; Revised: 22-Aug-2022, Manuscript No. IJPCBS-22-71204 (R); Published: 29-Aug-2022
Abstract
Background: Antiretroviral treatment (ART) is aimed for complete suppression of viral replication but fails for various reasons. This study aimed to determine the prevalence and associated factors of treatment failure among people on first line ART in Amhara region, Northeast Ethiopia.
Methods: A cross sectional study was conducted from March to October, 2018 among people of all age who came for HIV viral monitoring laboratory test in 16 randomly selected antiretroviral treatment sites. Sample size was calculated by using single population proportion formula proportionated to facilities and clients were recruited consecutively when they come to their viral load test. Questionnaire survey was taken focusing on demographic data and possible risk factors of antiretroviral treatment failure. Clinical history including baseline characteristics was extracted by reviewing medical records. Viral load test was done using real time HIV-1 viral load assay (Abbott Laboratories, USA) and data was analyzed using STATA version 14.
Results: A total of 640 clients (median age of 38 years ± SD=11.3 years) on first line antiretroviral treatment were enrolled. The overall treatment failure was 19.04% with clinical, immunologic and viral failures of 0.31%, 12.66% and 6.09% respectively. The viral suppression was 91.09% and re-suppression was 27.58%. Multivariable logistic regression analysis showed significance association of treatment failure with low baseline CD4 count ≤250 (AOR=2.16) and fair/poor adherence (AOR=3.41).
Conclusion: In conclusion the overall antiretroviral treatment failure in Amhara region, Ethiopia was 19.04%. Fair/poor adherence and low base line CD4 count are significantly independent predictors of treatment failure. Therefore proving client follow up to adherence and early testing and initiation of ART before decline of CD4 count should be strengthened. Higher proportion of viral failure in male study participants indicates the need for drug resistance survey in the study area as they are highly mobile.
Keywords
RAmhara region; ART failure; Dessie; Ethiopia; HIV; Viral suppression ART, Anti-Retroviral Treatment; AZT, Zidovudine; D4t, Satvudine; EFV, Efavirenz; (HIV) Human Immunodeficiency Virus; (PLHIV) People Living with HIV; (TB) Tuberculosis; (TDF) Tenofovir Deoxy Fumerate; (WHO) World Health Organization; 3TC Lamivudine
Introduction
Human Immunodeficiency Virus (HIV) is affecting more than 36.7 million people at the end of 2016 worldwide with 1.8 million people becoming newly infected globally in the same year and Africa is the most affected continent with 25.6 million people living with HIV which accounts for almost two-thirds of the global total of HIV infections. In Ethiopia central statistical agency (CSA) in the 2016 Ethiopia demographic and health survey (EDHS) reported the prevalence of HIV at 1.2% nationally ranging from 0.1% in Somali region to 4.8% in Gambella and 0.4% in rural areas versus 2.9% in urban areas that indicated urban areas are affected seven times higher than rural areas. Estimated number of people living with HIV and needing ART in Ethiopia are 710,000 in which about 650,000 (91%) are adults and 62,000 (9%) are children under 15 years of age. Antiretroviral treatment is recommended for everyone with HIV to help people with HIV live a longer, healthier life and to reduce the risk of HIV transmission. Ethiopia accepted the WHO recommendation to provide lifelong ART to all people living with HIV, including children, adolescents and adults, pregnant and breastfeeding women, regardless of clinical status or CD4 cell count. Antiretroviral treatment is aimed for complete suppression of viral replication and once effective ART is started, it usually takes 3 to 6 months for a person’s viral load to reach to undetectable level; which makes people living with HIV (PLHIV) less prone to HIV related illness. The UNAIDS 2020 target is to have 90% of the people living with HIV know their status, 90% of the people living with HIV (who know their HIV status as positive) are already on ART treatment, 90% of people on treatment are virally suppressed meaning successful treatment or undetectable viral load in two consecutive viral load measurements after 6 months of treatment. However, according to a recent global report, only 73% of PLHIV were virally suppressed or had successful antiretroviral treatment worldwide indicating the global target of 90% viral suppression is yet not being achieved and sub-Saharan Africa has a similar report [1].
Treatment failure (clinical failure, immunologic failure or viral failure, or any combination of the three) is an important indicator showing disease progression. Although viral failure is considered an early indicator of treatment success, clinical and immunologic conditions need to be described for better outcomes. Studies in Ethiopia showed regional variations in the magnitude of all types of ART failure, ranging from 4.1% [2] to 19.8% [3] and Amhara region is one of the high HIV burden regions with growing concern to reach the 3rd 90 due to gaps in ART programs [4]. Antiretroviral treatment fails for a variety of reasons most importantly non-adherence to antiretroviral treatment [5] which is higher in low and middle income countries [6]. Other factors that can contribute to treatment failure include antagonism between some drug combinations, degree of CD4 status before treatment initiation [7], presence of co-infection, type of treatment regimen, body mass index (BMI) [8], old age [9] and other socio economic factors. Therefore, describing the situation in the region may help to minimize unnecessary regimen switch, to improve current practices, thereby improving health outcomes and preventing emergence and transmission of drug resistant strains. In addition, prevention of treatment failure by identifying and minimizing factors related to the patients is relatively easy but has a greater impact in the fight against the diseases and inadequate information is found in the region and if present it used retrospective method that depends on secondary data which is mostly incomplete to get all necessary information related to the problem. Accordingly this study is aimed to assess the magnitude and associated factors related to treatment failure among people on first line ART in Amhara region, North east Ethiopia.
Materials and Methods
Study area and period
The study was conducted in the Amhara region, Ethiopia which consists 15 zonal administrative units with a 2017 estimated population of 21.1 million. According to the regional health bureau number of PLHIV were about 208,000 and the region has more than 300 ART site health facilities and 5 viral load monitoring laboratories. However, about 40% of PLHIV were found in 32 priority towns and we randomly selected 16 ART site health facilities (11 health centers and 5 hospitals) from 6 zonal administrations in the eastern part of the region were studied from March, to October, 2018.
Study design
A cross sectional study was conducted among first line antiretroviral initiated clients. A questionnaire survey using a pre-structured questionnaire was taken focusing on demographic data and possible risk factors of antiretroviral treatment failure. Clinical history including baseline characteristics and adherence status was extracted by reviewing medical records using data abstraction checklist. Viral load test was done using automated real time HIV-1 viral load sp2000 extraction and m2000 detection system (Abbott Laboratories, IL, USA) having a detection limit of 50 copies/ml. Those who have viral load result greater than 1000 copies/ml were appointed at 3 month for another viral load test to confirm viral failure. Immunologic failure is assessed by comparing baseline and current CD4 cell count where <100 cells/μL, below baseline, or <50% of peak after taking ART for 6 months is considered as immunologic failure. For children younger than 5 years, persistent CD4 levels below 200 cells/mm or <10% peak after 6 month of treatment or for children older than 5 years, persistent CD4 levels below 100 cells/mm was considered as immunologic failure.
Sample size and sampling technique
Sample size was determined using the formula for single population proportion by taking 19.8% prevalence of treatment failure. After considering a 2.5 design effect and 5% nonresponse a total of 640 study subjects were proposed and consecutively recruited when they attend the clinics for their first viral load follow up. Clients on ART visiting the health facilities for first viral load test during the study period were consecutively recruited for the study.
Operational definitions
• Viral suppression: a viral load of <1000 copies/ mL after 6 months of ART
• Viral re-suppression: a viral load of >1000 copies/mL after 6 months of ART and <1000 copies/mL after another 3 months of enhanced adherence and counseling.
• Viral failure: a repeated viral load of >1000 copies/mL after 3 months of the first viral load.
• Clinical failure: new or recurrent clinical event indicating severe immunodeficiency (WHO clinical stage 4 conditions) after 6 months of ART for adults and for Children new or recurrent clinical event indicating advanced or severe immunodeficiency (WHO clinical stage 3 and 4 clinical condition with exception of TB after 6 months of ART).
• Immunologic failure: For adults older than 19 years: CD4 cell count of <100 cells/μL, below baseline, or <50% of peak after 6 months of ART.
- For children older than 5 years: Persistent CD4 levels below 100 cells/mm
- For children younger than 5 years: Persistent CD4 levels below 200 cells/mm or <10%.
• Treatment failure: Presence of immunologic, viral or clinical failure or any combination of these.
Data quality assurance and analysis
Data collection and laboratory test was made by trained health professionals and standardized formats were used for data extraction from medical charts at each health facility. Data collection process was supervised by the investigators. Collected data was checked for completeness prior to data entry and data exploration on entered data was made to see unexpected values, outliers, and identify variables which need transformation. The data was analyzed by STATA version 14 (Statacorp, USA). Frequencies, proportion and summary statistics were used to describe the study population in relation to relevant variables. Odds ratio and P-value were used to assess the presence and degree of association between treatment failure and possible risk factors. Variables with p<0.20 in binary logistic regression were recruited to multivariable regression and P<0.05 was considered as presence of significant association.
Results
Socio-demographic and clinical characteristics
A total of 640 clients of all age were enrolled in the study from which 405 (63.28%) were females. The median age was 38 years (±SD=11.3 years). More than half of study participants, 374 (58.44%) were between 19 to 35 years of age and 172 (26.88%) were between 36 to 49 years old. Five hundred one (78.29%) of the study participants were below primary education and 337 (52.66%) were married (Table 1). The mean length of stay before treatment initiation was 8 month (+SD=19 month) ranging from <1 month to 120 months but from 124 participants who were tested after launching of test and treat strategy of the country 84 (67.74%) study participants were initiated within 1 month of testing. The mean baseline CD4 count was 282 cells/ mm3 (±238) and the current mean CD4 count was 515 cells/mm3 (±246) which showed significance increment from the baseline (p<0.001) and the CD4 change in females was significantly higher than in males (p<0.01) (Figure 1). As indicated in Table 2, only 223 (34.84%) study participants were categorized in WHO stage I at the start of antiretroviral treatment and 231 (36.09%) had been in WHO stage III. The current WHO staging indicated improvement of clinical status in which 590 (92.19%) were in WHO stage I. About half of study participants 287 (49.65%) stay less than 15 days to initiate ART after diagnosis. TDF-3TC-EFV combination was the most prescribed regimen during ART initiation which was 338 (52.81%) and it is still the most common in the current regimen comprising 378 (59.08%) of all other regimens. One hundred eighteen (18.44%) had history of regimen shift and 98 (15.31%) had history of TB treatment. Regarding their adherence, as measured by pill count and retrieved from their medical records, 513 (80.16%) was reported to have good adherence and nutritional status using body mass index (BMI) showed 237 (37.03%) were below 18.5 that is underweight.
| Characteristics | Frequency | Percent |
|---|---|---|
| Age | ||
| ≤ 18 years | 48 | 7.5 |
| 19-35 years | 374 | 58.44 |
| 36-49 years | 172 | 26.87 |
| ≥ 50 years | 46 | 7.19 |
| Education | ||
| No formal education | 260 | 40.63 |
| Primary school | 241 | 37.66 |
| Secondary school | 102 | 15.93 |
| College and above | 37 | 5.78 |
| Sex | ||
| Male | 235 | 36.72 |
| Female | 405 | 63.28 |
| Marital status | ||
| Married | 337 | 52.66 |
| Never Married | 106 | 16.56 |
| Divorced | 155 | 24.22 |
| Widowed | 42 | 6.56 |
Table 1: Socio demographic characteristics of clients on first line ART, Amhara region, Ethiopia, 2018.
| Characteristics | Frequency | Percent |
|---|---|---|
| WHO clinical stage | ||
| Stage I | 223 | 34.84 |
| Stage II | 155 | 24.23 |
| Stage III | 231 | 36.09 |
| Stage IV | 31 | 4.84 |
| CD4 Baseline | ||
| ≤100 | 126 | 19.69 |
| 101-200 | 148 | 23.12 |
| 201-350 | 201 | 31.41 |
| 351-500 | 87 | 13.59 |
| >500 | 78 | 12.19 |
| History of TB Treatment | ||
| Yes | 98 | 15.31 |
| No | 542 | 84.69 |
| Body Mass index(Kg/m2) | ||
| ≤ 18.5 | 237 | 37.03 |
| ≥ 18.6 | 403 | 62.97 |
| Adherence | ||
| Good | 513 | 80.16 |
| Fair/poor | 127 | 19.84 |
| Total | 640 | 100 |
Table 2: Baseline clinical characteristics of clients on first-line ART, Amhara region, Ethiopia, 2018.
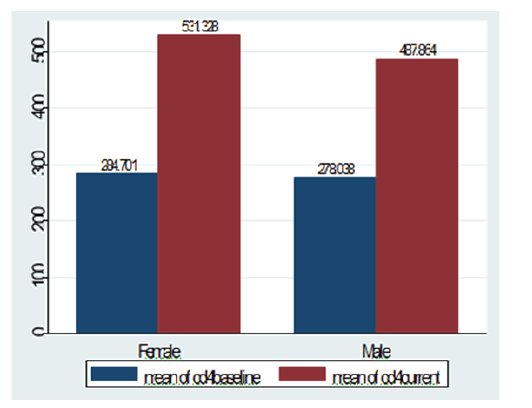
Figure 1: Baseline and current CD4 count among females and line ART in Amhara region, Ethiopia
Treatment failure and its associated factors
The overall treatment failure of first line antiretroviral drugs was 19.04% with clinical, immunologic and viral failure of 0.31%, 12.66% and 6.09% respectively and the treatment success was 80.94% (Figure 2). The viral suppression rate was 91.09% and re-suppression was achieved for 8 out of 28 (27.58%) study participants tested during the 3 months of enhanced adherence counseling follow up. Among 57 participants who had unsuppressed viral load (>1000 copies/ml at 6 month), more than half, 29 (50.88%) study participants dropout and considered as viral failure. High proportion of overall treatment failure, 33.33%, immunologic failure, 18.75%, as well as viral failure, 14.58%, was observed among age groups less than 18 years. As also indicated in Table 4, the odds of treatment failure in age group <18 years, from 19 to 35 and from 36 to 49 was 2.12, 1.16 and 1.20 times higher than the age group 50 years and above respectively. High proportion of immunologic failure was also observed among females (13.09%) but viral failure was high, 8.09%, in males (Table 3). Binary logistic regression was conducted for each predictor variable and those with p<0.20 (adherence, age and baseline CD4 count) were included in multivariable logistic regression analysis to explore factors associated with treatment failure and it showed baseline CD4 and adherence had significant association. Clients having less than 250 CD4 baseline count had 2.16 higher odds of treatment failure than their counter parts. It is found that fair/poor adherence had 3.41 times higher odds of treatment failure as compared to those with good adherence (Table 4).
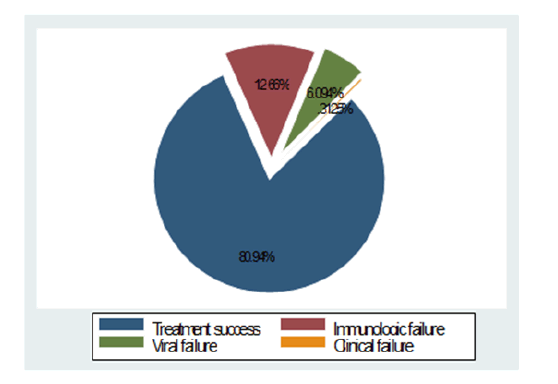
Figure 2: Antiretroviral treatment response of clients on first line ART in Amhara region, Ethiopia, 2018.
| Characteristics | Clinical failure N (%) | Immunologic failure N (%) | Viral failureN (%) | Overall treatment failure N (%) | Treatment success N (%) |
|---|---|---|---|---|---|
| Age | |||||
| ≤ 18 years | 0 (0.00) 2 | 9 (18.75) | 7 (14.58) | 16 (33.33) 69 | 32 (66.67) |
| 19-35 years | (0.53) 0 | 46 (12.30) | 21 (5.61) | 18.45 | 305 (81.55) |
| 36-49 years | (0.00)0 | 21(12.21) | 9 (5.23) 2 | 30 (17.44) | 142 (82.56) |
| ≥ 50 years | 0.00 | 5 (10.87) | 4.35 | 7 (15.22) | 39(84.78) |
| Sex | |||||
| Male | 1 (0.43) | 28 (11.91) | 19 (8.09) | 48 (20.43) | 187 (79.57) |
| Female | 1 (0.25) | 53 (13.09) | 20 (4.94) | 74 (18.27) | 331 (81.73) |
| Comorbidity | |||||
| Yes | 0 (0.00) | 23 (13.69) | 13 (7.74) | 36 (21.43) | 132 (78.57) |
| No | 2 (0.42) | 58 (12.29) | 26 (5.51) | 86 (18.22) | 386 (81.78) |
| Body mass index (Kg/m2 ) | |||||
| ≤ 18 | 1 (0.42) | 29 (12.24) | 15 (6.33) | 45 (18.99) | 192 (81.01) |
| >18 | 1 (0.25) | 52 (12.90) | 24 (5.96) | 77 (19.11) | 326 (80.89) |
Table 3: Proportion of first line treatment response in Amhara region, Ethiopia, 2018.
| Characteristics | Treatment failure | COR (95%CI) | AOR (95%CI) | p-value | |
|---|---|---|---|---|---|
| Yes N (%) | No N (%) | ||||
| Age | |||||
| <18 years | 16 (33.33) | 32 (66.67) | 2.78 (1.02-7.60) | 2.12 (0.74-6.01) | P=0.157 |
| 19-35 years | 69 (18.45) | 305 (81.55) | 1.26 (0.54-2.93) | 1.16 (0.48-2.78) | P=0.731 |
| 36-49 years | 30 (17.44) | 142 (82.56) | 1.17 (0.48-2.88) | 1.20 (0.47-3.03) | P=0.691 |
| >=50 years | 7 (15.22) | 39 (84.78) | 1 | 1 | |
| Baseline CD4 count | |||||
| <250 | 74 (24.34) | 230 (75.66) | 1.93 | 2.16 (1.41-3.30) | P<0.001 |
| >250 | 48 (14.29) | 288 (85.71) | 1 | 1 | |
| Adherence | |||||
| Fair/poor | 45 (35.43) | 82(64.57) | 3.10 (2.00-4.81) | 3.41 (2.16-5.38) | P<0.001 |
| Good | 77 (15.01) | 436 (84.99) | 1 | 1 | |
Table 4: Factors associated with treatment failure among people on first line ART in Amhara Region, Ethiopia, 2018
Discussion
This study was designed to assess the magnitude and associated factors of treatment failure from 16 health facilities in Amhara region. The study found 19.04% overall treatment failure to first line antiretroviral treatment. The majority of failure was indicated by immunologic failure (12.66%) followed by viral failures (6.09%) and clinical failure (0.31%). However, clients with baseline severe immunosuppression often take long to achieve immune recovery, even if virologic suppression occurs and immune reconstitution inflammatory syndrome (IRIS) might be the reasons that new or recurrent opportunistic conditions occur for clinical failure despite virologic responses to ART [10]. Therefore, for such clients, indication of treatment failure is suggested to depend on viral failure. The finding of viral failure in the present study is slightly higher than a study from Addis Ababa, Ethiopia which reported 4.4%, and another Ethiopian study that reported 1.3% viral failure which could be due to real difference in the local setting because of presence of different adherence status. However the finding is much lower than Ethiopian national viral failure of 11%.
Viral suppression of 91.09% after 6 months of ART was observed and the re-suppression for clients who had unsuppressed viral load was 27.58% which was lower than the Ethiopian national figure, 35.3%. However high rate of clients, 50.88%, dropout from enhanced adherence and counseling which is much higher than 19% dropout report from Ethiopia national study which will have great programmatic effect to achieve the third 90’s goal. The present study found baseline CD4 count and fair/poor adherence were significantly associated and independent predictors of treatment failure. Higher risk of treatment failure in those CD4 base line count below 250 was revealed in line with findings in Ethiopia [9,11]. Adherence is an important factor for treatment failure as it is used as indicator of weather the treatment failure is due to improper medication or due to viral factors such as resistance mutations. In our finding clients with fair/poor adherence were found to be 3.41 times higher risk of treatment failure compared to clients with good adherence similar to findings in different parts of Ethiopia [2,12] as well as studies in Kenya and Nigeria [13]. However in the present study clients data recorded by standard checklist assessment and self-report were used to describe adherence which might underestimate the actual scenario and the real situation may be high. In our finding the proportion of immunologic failure was higher in females than males, contrary to the proportion of viral failure which was about double higher among males than females (8.09 versus 4.94). This could be due to the situation in developing countries where many women living with HIV have limited access to health services that led them to late access of antiretroviral treatment. On the other hand higher viral failure in men participants that is 1.71 (95% CI: 0.85-3.44) higher odds of viral failure than women was observed consistent with studies in Uganda, Zimbabwe and Burkina Faso [14] which might be partially explained by adherence status or acquisition of resistant strain due to the mobile nature of this group. Although it is not statistically significant, this study showed that about 33.33% of overall treatment failure was observed among age group below 18 years followed by 19-35 years. This is supported by several studies in other parts of the country as well as studies from Kenya, Nigeria and Cameroon that revealed younger age groups were at risk of treatment failure which could be due to acquisition of drug resistance strains [11,15].
Conclusion
In this study we conclude that antiretroviral treatment failure in Amhara region, Ethiopia is
19.04%. Low CD4 baseline count and fair/poor adherence are significantly associated independent predictors of treatment failure. Therefore improving client follow up to adherence to treatment should be strengthened and ART program should focus on strengthening test and start strategy to enroll clients early before CD4 count declines. A higher proportion of viral failure in male study participants indicates the need for a drug resistance survey in the study area as they are highly mobile.
Limitation of the Study
The findings of this study have to be seen in light of some limitations. The use of adherence measurement based on health care provider’s assessment didn’t adequately detect poor adherence. In addition, impact of primary ART resistance to treatment failure and baseline viral load is not known as the test is not routinely available, thus, we suggest further large scale study.
Data Availability
The dataset used to support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
The study has got approval from Amhara Public Health Institute ethical review board ref. number 04/018/2010. All study subjects during the study period was informed the purpose of the study and their consent was sought in written.
Acknowledgement
The authors thank Amhara Public Health Institute for supporting this work. Our special gratitude goes to Azmera Kebede for her support and staffs of health facilities that participate in the study and all study participants
Author's Contribution
Minwuyelet Maru: Designed the study, analyzed and interpreted the data, Initiated and drafted the manuscript, Daniel Dagne: Analyzed the data, Addisu Tesfie: Involved in proposal write up and data collection, Asefa Missaye: revised the manuscript, Gizachew Yismaw: Revised the manuscript, Andargachew Mulu: Revised the manuscript
Funding
This work was supported by the Amhara Public Health Institute core budget.
Disclosure
The authors declare that they have no competing interests.
References
- Ayele TA, Worku A, Kebede Y, Alemu K, Kasim A, Shkedy Z. Choice of initial antiretroviral drugs and treatment outcomes among HIV-infected patients in sub-Saharan Africa: Systematic review and meta-analysis of observational studies. Syst Rev 2017; 6:173.
[Crossref] [Google Scholar] [PubMed]
- Ayalew MB, Kumilachew D, Belay A, Getu S, Teju D, Endale D, Tsegaye Y, Wale Z. First-line antiretroviral treatment failure and associated factors in HIV patients at the University of Gondar Teaching Hospital, Gondar, North west Ethiopia. HIV/AIDS 2016; 8:141-146.
[Crossref] [Google Scholar] [PubMed]
- Teshome Yimer Y, Yalew AW. Magnitude and predictors of Anti-Retroviral Treatment (ART) Failure in private health facilities in Addis Ababa, Ethiopia. PLoS ONE 2015; 10:e0126026.
[Crossref] [Google Scholar] [PubMed]
- Worku ED, Asemahagn MA, Endalifer ML. Epidemiology of HIV infection in the Amhara region of Ethiopia, 2015 to 2018 surveillance data analysis. HIV/AIDS 2020; 12:307-314.
[Crossref] [Google Scholar] [PubMed]
- El-Khatib Z, Katzenstein D, Marrone G, Laher F, Mohapi L, Petzold M, Morris L, Ekström AM. Adherence to drug-refill is a useful early warning indicator of virologic and immunologic failure among hiv patients on first-line aRT in South Africa. PLoS ONE 2011; 6:e17518.
[Crossref] [Google Scholar] [PubMed]
- Nachega JB, Mills EJ, Schechter M. Antiretroviral therapy adherence and retention in care in middle-income and low-income countries: Current status of knowledge and research priorities. Curr Opin HIV AIDS 2010; 5:70-77.
[Crossref] [Google Scholar] [PubMed]
- Gilks CF, Walker AS, Munderi P, Kityo C, Reid A, Katabira E, Goodall RL, Grosskurth H, Mugyenyi P, Hakim J, Gibb DM; DART Virology Group and Trial Team. A Single CD4 Test with 250 Cells/Mm threshold predicts viral suppression in HIV-infected adults failing first-line therapy by clinical criteria. PLoS ONE 2013; 8:e57580.
[Crossref] [Google Scholar] [PubMed]
- Babo YD, Alemie GA, Fentaye FW. Predictors of first-line antiretroviral therapy failure amongst HIV-infected adult clients at Woldia Hospital, Northeast Ethiopia. PLoS ONE 2017; 12:e0187694.
[Crossref] [Google Scholar] [PubMed]
- Bosch RJ, Bennett K, Collier AC, Zackin R, Benson CA. Pretreatment factors associated With 3-Year (144-Week) virologic and immunologic responses to potent antiretroviral therapy. Acquir Immune Defic Syndr 2007; 44:268-277.
[Crossref] [Google Scholar] [PubMed]
- Smith K, Kuhn L, Coovadia A, Meyers T, Hu CC, Reitz C, Barry G, Strehlau R, Sherman G, Abrams EJ. Immune reconstitution inflammatory syndrome among HIVinfected South African infants initiating antiretroviral therapy. AIDS 2009; 23:1097-1107.
[Crossref] [Google Scholar] [PubMed]
- Bayu B, Tariku A, Bulti AB, Habitu YA, Derso T, Teshome DF. Determinants of virological failure among patients on highly active antiretroviral therapy in University of Gondar Referral Hospital, Northwest Ethiopia: A case control study. HIV/AIDS 2017; 9:153-159.
[Crossref] [Google Scholar] [PubMed]
- Haile D, Takele A, Gashaw K, Demelash H, Nigatu D. Predictors of treatment failure among adult antiretroviral treatment (ART) Clients in Bale Zone Hospitals, South Eastern Ethiopia. PLoS ONE 2016; 11:e0164299.
[Crossref] [Google Scholar] [PubMed]
- Anude CJ, Eze E, Onyegbutulem HC, Charurat M, Etiebet MA, Ajayi S, Dakum P, Akinwande O, Beyrer C, Abimiku A, Blattner W. Immuno-virologic outcomes and immuno-virologic discordance among adults alive and on anti-retroviral therapy at 12 months in Nigeria. BMC Infect Dis 2013; 13:113.
[Crossref] [Google Scholar] [PubMed]
- Penot P, Héma A, Bado G, Kaboré F, Soré I, Sombié D, Traoré JR, Guiard-Schmid JB, Fontanet A, Slama L, Bruno Sawadogo A, Laurent C. The vulnerability of men to virologic failure during antiretroviral therapy in a public routine clinic in Burkina Faso. J Int AIDS Soc 2014; 17:18646.
[Crossref] [Google Scholar] [PubMed]
- Babo YD, Alemie GA, Fentaye FW. Predictors of first-line antiretroviral therapy failure amongst HIV-infected adult clients at Woldia Hospital, Northeast Ethiopia. PloS one2017; 12:e0187694.
[Crossref] [Google Scholar] [PubMed]

