Research Article - International Journal of Pharmaceutical, Chemical and Biological Sciences ( 2021) Volume 11, Issue 2
ALPHA AMYLASE, ALPHA GLUCOSIDASE AND GLUCOSE DIFFUSION INHIBITORY ACTIVITY OF VIDANGADI KVATHA
Minshu Prashant*, Sanjay Kumarn and B. RamMinshu Prashant, Department of Dravyaguna, Banaras Hindu University, India,
Published: 20-Oct-2021
Abstract
Diabetes mellitus type 2 refers to a group of metabolic disorder that is characterized by hyperglycemia. Diabetes mellitus type 2 is caused by either deficiency in production of insulin by the pancreas or insulin resistance. In India, this increase is estimated to be 58%, from 51 million people in 2010 to 87 million in 2030. An in vitro a-amylase enzyme inhibition test of Vidangadi Kvatha was performed. The results show that the IC50 value of Vidangadi Kvatha and Acarbose was 70.39 ± .0890 μg/ ml and 40.89 ± 7.162 μg/ml. The α-glucosidase inhibitory activity of the Vidangadi Kvatha was evaluated in triplicate using standard method. The % inhibition and IC50 value of Vidangadi Kvatha and Acarbose were calculated from the graph plotted between % inhibition and concentration of Vidangadi Kvatha. The IC50 value of Vidangadi Kvatha and Acarbose was found 321 μg/ml and 175 μg/ml. In glucose diffusion inhibitory assay percent glucose diffusion retardation index (% GDRI) was calculated and the result shows the % GDRI was found maximum in Vidangadi Kvatha i.e. 45% at 30 min time interval and 83% at 60 min time interval in comparison to other drugs. The present study focus on antidiabetic property of Vidangadi Kvatha and trying to established the mechanism of action through different experimental models like inhibition of amylase activity, glucosidase enzyme activity, glucose diffusion inhibitory activity.
Introduction
Diabetes mellitus refer to a group of common metabolic disorder that shares the phenotype of hyperglycemia. Diabetes mellitus is a chronic disorder and it is caused by inherited and/or acquired deficiency in production of insulin by the pancreas, or by the ineffectiveness of the insulin produced, i.e. insulin resistance. This leads into increased concentrations of glucose in the blood, which in turn damage many of the body’s systems, in particular the blood vessels and nerves [1]. The number of people with diabetes has been raised from 108 million in 1980 to 422 million in 2014. The global prevalence of diabetes among adults over 18 years of age has been raised from 4.7% in 1980 to 8.5% in 2014. Diabetes prevalence has been rising more rapidly in middle and low income countries. Diabetes is a major cause of blindness, kidney failure, heart attacks, stroke and lower limb amputation [2]. In 2015, an estimated 1.6 million deaths were directly caused by diabetes. Almost half of all deaths attributable to high blood glucose occur before the age of 70 years. WHO projects that diabetes will be the seventh leading cause of death in 2030. Diabetes might be one of the most talked about diseases across the world and especially in India. In India, today have more people with type 2 diabetes (more than 50 million) than any other Nation. In India, this increase is estimated to be 58%, from 51 million people in 2010 to 87 million in 2030 [3]. Several distinct type of diabetes exist and are caused by a complex interaction of genetics and environmental, factor contributing to hyperglycemia include reduce insulin secretion, decrease glucose utilization and increase glucose production. Diabetes mellitus is the leading cause of end stage renal disease (ESRD), Non traumatic lower extremity amputation because of diabetic peripheral neuropathy (DPN). Common presenting symptom of Diabetes mellitus included polyurea, polydipsia, polyphagia weight loss, fatigue, weakness, blurred vision, frequent superficial infection and poor wound healing [4]. In comparative to synthetic drugs, herbal drugs are effective, less side effects, broad range of action and relatively low cost, makes polyherbal formulation is a good choice. Besides the miraculous achievement of modern medical science, humanity is passing through a horror of disease and drug phobia, among the several health problems, Diabetes mellitus is a giant disease considered as one of the arch enemy of the mankind [5]. Diabetes and its complications pose a major threat to future public health resources throughout the world [6]. Changing life style, lack of exercise, fast foods, improper unbalanced high fat low fiber diet, and sedentary lifestyle are showing upward trend in India. This has led to the emergence of Diabetes Mellitus in the region. In Ayurveda, Madhumeha is nearest clinical entity for Diabetes Mellitus. Ayurveda has emphasized on preventive, promotive measures with due consideration of appropriate ahara and vihara. They categorized diabetes into 2 groups the obese and the lean and prescribed tikth rasa pradhana dravya and Shakha for treatment and strenuous exercises for the obese diabetics. Astanga Hridayakara while explaining treatment for nirdhan rogi told to walk for 100 yojanas bare foot and to eat like muni [7].
The determination of α-amylase inhibition was carried out by quantifying the reducing sugar liberated under the assay conditions. α-amylase hydrolyzes carbohydrates by acting on terminal, non-reducing α (1→4) linked D-glucose residues with the release of D-glucose. D The enzyme inhibitory activity was expressed as a decrease in units D-glucose liberated [8]. Alpha-glucosidase inhibitors are saccharides that act as competitive inhibitors of enzymes needed to digest carbohydrates: specifically alpha-glucosidase enzymes in the brush border of the small intestines. The membrane-bound intestinal alpha-glucosidase hydrolyzes oligosaccharides, trisaccharides, and disaccharides to glucose and other monosaccharides in the small intestine. Acarbose also blocks pancreatic alpha-amylase in addition to inhibiting membrane-bound alpha-glucosidases. Pancreatic alpha-amylase hydrolyzes complex starches to oligosaccharides in the lumen of the small intestine. Inhibition of these enzyme systems reduces the rate of digestion of carbohydrates. Less glucose is absorbed because the carbohydrates are not broken down into glucose molecules. In diabetic patients, the short term effect of these drugs therapies is to decrease current blood glucose levels: the long term effect is a small reduction in hemoglobinA1c level [9]. An α-glucosidase is a membrane bound enzyme located at the epithelium of small intestine, and the key enzyme of carbohydrate digestion. It specifically hydrolyzes the α-glucopyranoside bond, thereby releasing α-D-glucose from the non-reducing end of sugar, α-Glucosidase hydrolyzes the terminal, non-reducing 1,4-linked α-D-glucose residues with release of α-D-glucose. α-glucosidase is needed by all animals to hydrolyze glucose. α-D-glucosidase plays a role in the conversion of carbohydrates into glucose [10]. Glucose diffusion inhibitory assay is a simple diffusion method to evaluate the glucose movement in vitro and is expressed in terms of glucose diffusion retardation index (GDRI) [11]. This method involves the use of a sealed dialysis membrane into which solution of glucose and NaCl was introduced and the appearance of glucose in the external solution was measured. The dialysis membrane was sealed at each end and placed in a centrifuge tube. The tubes were placed on an orbital shaker and kept at room temperature. The movement of glucose into the external solution was monitored at set time intervals [12].
The proposed research work has also been launched with the same view to explore the anti-diabetic effects of Vidangadi Kvatha. (Polyherbal formulation) by searching different literatures starting from Veda to the Ayurvedic pharmacopeia of India (API) to formulate out a drug which act as anti-hyperglycemic or antidiabetic.
Requirements
Alpha amylase enzyme (SRL Mumbai), Alpha glucosidase enzyme (SRL Mumbai), Gly-pronitroanaline (GPPN) (SRL Mumbai), Acarbose, 3,5-dinitrosalicylic acid (DNSA) (SRL Mumbai), Tris-acetate buffer (Merk Mumbai), Starch, P-Nitro-phenyl-α-D-Glucopyranoside, D-glucose, glucose oxidase, dialysis membrane, Vidangadi Kvatha, Vidanga (Fruit), Haridra (Rhizomes), Shunthi (Rhizomes), Yashtimadhu (Stem), Gokshura (Fruit).
Methodology
Preparation of decoction of Vidangadi Kvatha
The ingredients of Vidangadi Kvatha were Vidanga (Embelia ribes Burm F.) [13,14], Haridra (curcuma longa) [15-19], Shunthi (zingiber officinale Rosc) [20-23], Yashtimadhu (Glycrrhiza glabra Linn.) [24- 28], Gokshura (Tribulus terrestris Linn) [29-32]. All ingredients were subjected for size reduction using the pulvarizer. Equal amount of all crude drugs was soaked in 4 times water in vessel and kept overnight for 12 hrs. After 12 hrs contents were boiled at 90°C-95°C with stirring. Water was evaporated till 1/4th amount was remains and galenicals was filtered through cotton cloth. Filtrate was dried with rotatory evaporator and dried powder was used for quality control standard test and in vitro antidiabetic activity (Table 1).
| SN | TEST | OBSERVATION |
|---|---|---|
| 1 | Description | Kvatha powder is dark brown in color, astringent-sweet taste and pungent smell |
| 2 | Test for heavy metal | Mercury – BDL (Below detectable limit) Arsenic – BDL (Below detectable limit) Lead – BDL (Below detectable limit) Cadmium – BDL (Below detectable limit) |
| 3 | Test for microbes | S. aureus - nil Pseudomonas aeruginosa - nil E. coli - nil Salmonella spp. - nil |
| 4 | Pesticide residue | Nil |
Table 1: Quality control testing of Vidangadi Kvatha.
Physicochemical evaluation of Vidangadi Kvatha (Decoction)
Physicochemical evaluation of Vidangadi Kvatha (Decoction)
Phytochemical analysis of Vidangadi Kvatha (Decoction)
The Phytochemical screening of different extracts of crude drugs and Kvatha (decoction) were performed according to the procedure mentioned in Ayurvedic Pharmacopoeia of India (API).
The Phytochemical analysis of the different extracts was given below
Tests for carbohydrate
Fehling’s test: Equal amount of Fehling’s A solution (Copper sulphate in distilled water) and Fehling’s B solution (Potassium tartrate and sodium hydroxide in distilled water) reagent were mixed properly and few drops of it was added in extract and boiled and finally observed for presence of brick red precipitate of cuprous oxide which indicated the presence of carbohydrate.
Benedict’s test: 2 ml of the extract is treated with drops of Benedict reagent. Red or brown colored precipitate indicates the presence of carbohydrates.
Molisch’s test: 2 ml of extract was taken in a test tube and added 2 drops of a freshly prepared 20% alcoholic solution of β-naphthol and mix. 2 ml of conc. Sulphuric acid was added from the side of the test tube to form a violet ring which disappears on addition of an excess of alkali solution indicates the presence of carbohydrates.
Tests for Alkaloids
Dragendorff’s test: A few mg of extract of the drug was added in 5 ml of distilled water, followed by addition of 2 ml hydrochloric acid until an acid reaction occurs, then added 1 ml of Dragendorf’s reagent, an orange precipitate is produced immediately, occurrence of orange-red precipitate indicates the presence of alkaloid.
Mayer’s test: Added a few drops of Mayer’s reagent to 1 ml of acidic aqueous extract of the drug, pale-yellow precipitate is formed and appearance of buff-colored precipitate will be an indication for the presence of alkaloids.
Hager’s test: 1 ml of extract was taken in test tube and few drops of Hager’s reagent were added. Formation of yellow precipitate indicates the presence of alkaloids.
Wagner’s test: Acidify 1 ml of extract with 1.5 ml of hydro-alcoholic acid then added few drops of wagner’s reagent. A yellow of white precipitate was formed indicates the presence of alkaloids.
Tests for Sterol and Triterpenoids
2 ml of extract was mixed with few drops of chloroform and few drops of concentrated sulphuric acid and it was shaken well and allowed to stand for some time to observe yellow color precipitation indicated the presence of triterpenoids.
Detection of proteins (Biuret test)
2 ml of the extract was treated with 10% sodium hydroxide solution and 2 drops of 0.1% copper sulphate solution was added and observed for appearance of violet pink color which indicates the presence of proteins.
Detection of saponins 2 ml of alcoholic and aqueous extracts were mixed separately with 20 ml of distilled water and shake in a graduated cylinder for 15 mins. 1 cm layer of foam indicates presence of saponins.
Detection of phenolic compound and Tannins
A small amount of alcoholic and aqueous extracts were dissolved separately in water and was tested for the presence of phenolic compounds and tannins by adding few drops of dilute ferric chloride and lead acetate solution and the color of the solution was observed. Greenish black color indicates the presence of tannins.
Test for Flavonoids Ferric chloride test: Test solution (2 ml of extract) was treated with few drops of Ferric chloride solution. The yellow color of the solution was observed, if the solution turns to blackish red color indicates the presence of Flavonoids.
iShinoda’s test: Took 0.5 ml of extract in a test tube and 5-10 drops of dil. Hydrochloric acid was added followed by addition of small piece of magnesium. The formation of pink, reddish pink or brown color indicates the presence of flavonoids.
Test for glycosides: Detection of glycosides on paper spray solution No. 1 (0.5% aqueous solution of sodium metaperiodate) and waiting for 10 minutes after then spraying solution No. 2 (0.5% benzidine) (w/v) in solution of ethanol-acetic acid (4:1), white spot with blue black background shows presence of glycosides.
Test for steroids
Salkowaski Reactions: Added 1 ml of conc. Sulphuric acid to 2 ml of test solution from the side of the test tube, a red color was produced indicates the presence of steroids.
Liebermann-Burchard’s test: Added 2 ml of acetic anhydride solution to 1 ml extracts in chloroform followed by 1 ml of conc. Sulphuric acid. A greenish color is developed which turns to blue indicates the presence of steroids [33].
Alpha amylase inhibitory assay of Vidangadi Kvatha
The α-amylase enzyme inhibitory assay was performed using the DNSA method. The Vidangadi Kvatha (dry powder) was dissolved in prepared 10% DMSO and was further dissolved in prepared buffer solution ((Na2HPO4/NaH2PO4 (0.02 M), NaCl (0.006 M) at pH 6.9) to prepare different concentrations of 10 to 1000 μg/ml. 200 μl of α-amylase enzyme solution (2 unit/ml) was added in each test-tube containing 200 μl solution of Vidangadi Kvatha from the prepared stock solution of different concentration and test tubes was incubated for 10 min (at 30°C). After that 200 μl of starch solution (1% solution in water (w/v)) was added and incubated for 3 min. 200 μl DNSA reagent was added for reaction termination and test tubes was boiled at 85–90°C for 10 min on water bath. The final reaction mixture was cooled and diluted with addition of 5 ml of water and the absorbance of each test tube mixture was recorded at 540 nm with UV-Visible spectrophotometer. The blank solution of 100% enzyme activity was prepared. A blank solution was prepared with the Vidangadi Kvatha of each concentration free from enzyme solution. A positive control solution containing standard Acarbose (2 μg/ml–100 μg/ml) was prepared and reacted with Vidangadi Kvatha. The % α-amylase enzyme inhibition value was plotted against concentration of Vidangadi Kvatha and IC50 value was found from the prepared graph [34-36]. The α-amylase enzyme inhibitory property was expressed as % inhibition and it was calculated with the formula given below;
% α amylase inhibition=100 [(Abs100% control−AbsSample )/Abs100% Control)]
Alpha glucosidase inhibitory assay of Vidangadi Kvatha
This assay was carried out to investigate the in vitro inhibitory activity of Vidangadi Kvatha. The α-glucosidase inhibitory activity of the Vidangadi Kvatha was evaluated using a method described by Kim et al. with minor modifications. The reaction mixture containing 5 μl of the sample dissolved in DMSO at various concentrations and 495 μl of 100 mM phosphate buffer (pH 7.0) was added to 250 μl of 3 mM substrate solution p-Nitrophenyl-α-D-Glucopyranoside (PNPG). The mixtures were pre-incubated for 5 min at 37°C and 250 μl of α-glucosidase (0.065 unit mL-1) was added to start the reaction. The incubation was continued for 15 min and the reaction was terminated by the addition of 1 mL of 0.1 M sodium carbonate. Enzymatic activity was quantified by measuring the released product of p-Nitrophenyl at 400 nm. Acarbose was used as a positive standard [37,38] (Figure 1).
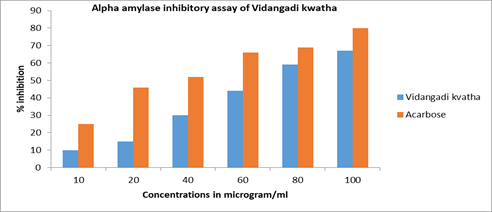
Figure 1: % inhibition against concentration in alpha amylase inhibitory assay of Vidangadi Kvatha and Acarbose
% α glucosidase inhibition=100 [(Abs100% control− AbsSample)/Abs100% Control]
Glucose diffusion inhibitory assay of Vidangadi Kvatha
A glass tube one sided sealed with dialysis membrane, filled with 2 ml of D D-glucose (22 mM prepared in 0.15 M NaCl) and 1 ml Vidangadi Kvatha. The open end of the glass tube was sealed with dialysis membrane. Glass tube placed in a beaker containing 45 ml of NaCl (0.15 M). The beaker was placed on an orbital shaking incubator (speed of 100 rotations per min at 37°C). Withdraw 10 ml Aliquot of the external solution at different time intervals. Solution was tested by glucose oxidase kit for investigating the presence of glucose in external solution. A Standard curve of glucose solution was plotted. The % glucose diffusion retardation index (GDRI) was calculated with the given formula [39]:

Results
Physicochemical evaluation of Vidangadi Kvatha
Vidangadi Kvatha powder was dark brown in color, astringent-sweet taste and pungent smell. Phytochemical screening of Vidangadi Kvatha was performed and found that carbohydrate, flavonoids, alkaloids, proteins, tannins saponins and amino acids was present in Kvatha. The volatile oils and steroids are absent in Vidangadi Kvatha. The microbial contamination, presence of heavy metals and pesticide residue was absent in Vidangadi Kvatha.
Alpha amylase inhibitory assay
An in vitro a-amylase enzyme inhibition test of Vidangadi Kvatha was performed in triplicate. The results show that the IC50 value of Vidangadi Kvatha and Acarbose was 70.39 ± 0.0890 μg/ml and 40.89 ± 7.162 μg/ ml. These results confirm that Vidangadi Kvatha have α-amylase inhibitory property (Figure 2).
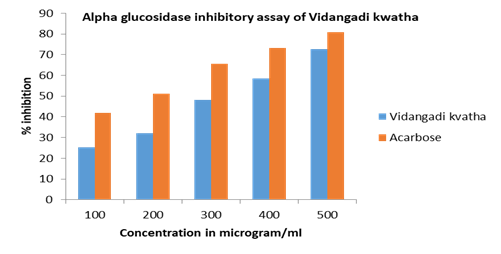
Figure 2: % inhibition against concentration in alpha glucosidase inhibitory assay of Vidangadi Kvatha and Acarbose
Alpha glucosidase inhibitory assay
The α-glucosidase inhibitory activity of the Vidangadi Kvatha was evaluated in triplicate using standard method. The % inhibition and IC50 value of Vidangadi Kvatha and Acarbose were calculated from the graph plotted between % inhibition and concentration of Vidangadi Kvatha. The IC50 value of Vidangadi Kvatha and Acarbose was found 321 microgram/ml and 175 microgram/ml. The result shows that Vidangadi Kvatha has alpha glucosidase inhibitory property (Table 2).
| S.N. | Enzymes | Drugs | Concentrations (µg/Ml) | Percentage of inhibition | IC50 value |
|---|---|---|---|---|---|
| 1. | Alpha amylase | Vidangadi Kvatha | 10 | 10 | 70.39±1.0890 |
| 20 | 15 | ||||
| 40 | 30 | ||||
| 60 | 44 | ||||
| 80 | 59 | ||||
| 100 | 67 | ||||
| Acarbose | 10 | 23 | 40.89±7.1625 | ||
| 20 | 46 | ||||
| 40 | 52 | ||||
| 60 | 66 | ||||
| 80 | 69 | ||||
| 100 | 80 | ||||
| 2. | Alpha Glucosidase | Vidangadi Kvatha | 100 | 25.2 | 321.73±20.33 |
| 200 | 32.2 | ||||
| 300 | 48.3 | ||||
| 400 | 58.4 | ||||
| 500 | 72.6 | ||||
| Acarbose | 100 | 42 | 175.99±15.91 | ||
| 200 | 51.1 | ||||
| 300 | 65.7 | ||||
| 400 | 73.2 | ||||
| 500 | 80.9 | ||||
| All values are present in Mean±SD, analyzed by one way ANOVA, P<0.05 | |||||
Table 2: The % inhibition and IC50 value of Vidangadi Kvatha and Acarbose against alpha amylase and alpha glucosidase enzyme.
Glucose diffusion inhibitory assay
In glucose diffusion inhibitory assay percent glucose diffusion retardation index (%GDRI) was calculated and the result shows the % GDRI was found maximum in Vidangadi Kvatha i.e. 45% at 30 min time interval and 83% at 60 min time interval (Figure 3) in comparison to other drugs (Table 3).
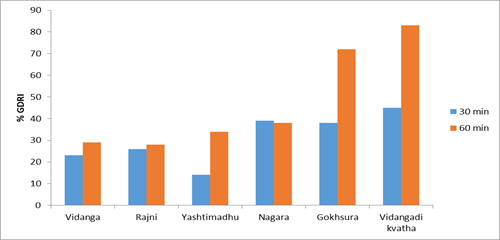
Figure 3: Graphical representation of the glucose concentration at 30 and 60 min in glucose diffusion inhibitory assay
| Test samples (160 mg/ml) | Glucose concentration in external solution at 30 min time intervals | Glucose concentration in external solution at 60 min time intervals | %GDRI at 30 min | %GDRI at 60 min |
|---|---|---|---|---|
| Control | 0.905 ± .001 | 1.366 ± 0.468 | 00 | 00 |
| Vidanga | 0.787 ± .004 | 0.821 ± 0.152 | 23 | 29 |
| Haridra | 0.679 ± 0.012 | 0.709 ± 0.044 | 26 | 28 |
| Yashtimadhu | 0.668 ± 0.016 | 0.628 ± 0.057 | 14 | 34 |
| Shunthi (Nagara) | 0.641 ± 0.010 | 0.527 ± 0.099 | 39 | 38 |
| Gokhsura | 0.744 ± 0.013 | 0.508 ± 0.037 | 38 | 72 |
| Vidangadi Kvatha | 0.462 ± 0.046 | 0.332 ± 0.041 | 45 | 83 |
| All values are expressed in mean ± SD | ||||
Table 3: Results of %GDRI value of Vidangadi Kvatha and Acarbose in glucose diffusion inhibitory assay.
Discussion
Ayurvedic medicines are most popular in the world because of its effectiveness, low cost and minimum side effects as well as the availability in the world and in India. In India there are about 45,000 plant species and 20,000–25,000 plants have medicinal properties. The ingredients of Vidangadi Kvatha was Vidanga (Embelia ribes Burm F.) Haridra (curcuma longa), Shunthi (zingiber officinale Rosc), Yashtimadhu (Glycrrhiza glabra Linn.) Gokshura (Tribulus terrestris Linn). The Vidangadi Kvatha was tested for the presence of microbes in preparation; presence of heavy metals (mercury, arsenic, cadmium and lead) and the results shows the absence of microbes and heavy metals. The pesticides was also absent in the preparation.
The physical property of Vidangadi Kvatha include there are yellowish-brown in color, pungent smell, light sweet in taste due to the presence of huge amount of tannins and flavonoids. The Phytochemical study was performed for estimation of Phytochemicals present in Kvatha and proved by chromatographic study. In in-vitro study Vidangadi Kvatha was evaluated for alpha amylase inhibition, alpha glucosidase inhibition, and glucose diffusion retardation from dialysis membrane. The alpha amylase inhibitory assay of Vidangadi Kvatha was performed as per standard protocol using Acarbose as standard and % inhibition and IC50 (Vidangadi Kvatha 70.39 ± 1.08 μg/ml, Acarbose 40.89 ± 7.16 μg/ml) was calculated. The IC50 value of Vidangadi Kvatha was found to be 321.73 ± 20.33 μg/ml and 175.99 ± 15.91 μg/ml for Acarbose the results are highly significant. In glucose diffusion inhibitory assay percent glucose diffusion retardation index (%GDRI) was calculated and the result shows the %GDRI was found maximum in Vidangadi Kvatha i.e. 45% at 30 min time interval and 83% at 60 min time interval in comparison to other drugs.
Conclusion
The present study focus on antidiabetic property of Vidangadi Kvatha and trying to established the mechanism of action through different experimental models like inhibition of amylase activity, glucosidase enzyme activity, glucose diffusion inhibitory activity.
Acknowledgement
A very thankful to Banaras Hindu University.
Conflict of Interest
No conflict of interest.
References
- Billings LK and Florez JC. The genetics of type 2 diabetes: what have we learned from GWAS. Ann N Y Acad Sci 2010; 1212:59-77.
- Lane MA. The cytological characters of the areas of Langerhans. Ameri J Anat 2005; 7:409-422.
- Butler AE, Janson J and Bonner-Weir S. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diab 2003; 52:102-110.
- Mcculloch DK, Raghu PK and Johnston C. Defects in Ã?-cell function and insulin sensitivity in normoglycemic streptozocin treated baboons: a model of preclinical insulin-dependent diabetes. J Clin Endocrinol Metab 1988; 67:785-792.
- Kendall DM, Sutherland DE and Najarian JS. Effects of hemipancreatectomy on insulin secretion and glucose tolerance in healthy humans. N Engl J Med 1990; 322:898-903.
- Cerasi E and Luft R. The plasma insulin response to glucose infusion in healthy subjects and in diabetes mellitus. Acta Endocrinol 1967; 55:278-304.
- Polonsky KS, Given BD and Hirsch LJ. Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. N Engl J Medicine 1988; 318:1231-1239.
- Abdullah N and Kasim KF. In Vitro Antidiabetic Activity of Clinacanthus nutans Extracts. Int J Pharmaco Phytochem Res 2017; 9:846-852.
- Wickramaratne MN, Punchihewa JC and Wickramaratne DBM. In Vitro alpha amylase inhibitory activity of the leaf extract of Adenanthera pavonina. BMC Complement Altern Med 2016; 16:466-471.
- Gawade Bhimraj and Farooqui Mazahar. Alphaâ??amylase Inhibitory Assay of Argemone Mexicana L. Leaves. Protein Sci 2017; 9:25-29.
- Butler AE, Janson J and Bonner-Weir S. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabet 2003; 52:102â??110.
- Bhandari U and Ansari MN. Ameliorative effect of an ethanol extract of Embelia ribes fruits on isoproterenol-induced cardiotoxicity in diabetic rats. Pharma Biol 2009; 47:669-674.
- Krishnan G, Ramesha KP, Sarkar M, Chakravarty P, Kataktalware MA and Saravanan B. Modified temperature humidity index for yaks. C Ind J Ani Sci 2009; 79:788-790.
- Ansari MN and Bhandari U. Antihyperhomocysteinemic activity of an ethanol extract from Embelia ribes in albino rats. Pharma Biol 2008; 46:283-287.
- Aggarwal BB, Kumar A and Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 2003; 23:363-398.
- Venkatesan P and Rao MN. Structure activity relationships for the inhibition of lipid peroxidation and the scavenging of free radicals by synthetic symmetric curcumin analogues. J Pharm Pharmacol 2000; 52:1123-1128.
- Vlietinck A, de Bruyne T, Apers S and Pieters L. Plant-derived leading compounds for chemotherapy of HIV infection. Planta Med 1998; 64:97-109.
- Wahl H, Tan L, Griffith K, Choi M and Liu JR. Curcumin enhance Apo2L/TRAILinduced apoptosis in chemo-resistant ovarian cancer cells. Gynecol Oncol 2007; 105:104-112.
- Yu ZF, Kong LD and Chen Y. Antidepressant activity of aqueous extracts of Curcuma longa in mice. J Ethnopharmacol 2002; 83:161-165.
- Zhang HG, Kim H, Liu C, Yu S, Wang J, Grizzle WE and et al. Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim Biophys Acta 2007; 1773:1116-1123.
- Abdullah N, Saat NZM, Hasan HA, Budin SB and Kamaralzaman S. Protective effect of the ethanol extract of Zingiber officinale Roscoe on paracetamol induced hepatotoxicity in rats. J Sains Kesihatan Malaysia 2004; 2:85-95.
- Akhila A and Tewari R. Chemistry of ginger: A review. Curr Res Med Arom Plants 1984; 6:143-156.
- Zhao YM, Zhang ML, Shi QW and Kiyota H. Chemical Constituents of Plants from the Genus Inula. Chem Biodiver 2006; 3:371-38.
- Ali BH, Blunden G, Tanira MO and Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem Toxicol 2008; 46:409-420.
- Isbrucker RA and Burdock GA. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.) its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul Toxicol Pharmacol 2006; 46:167-192.
- Badr SEA, Sakr DM, Mahfouz SA and Abdelfaterrestris tribulusah MS. Licorice (Glycyrrhiza glabra L.): Chemical composition and biological impacts. Res J Pharm Biol Chem Sci 2013; 4:606-621.
- Genovese T, Menegazzi M, Mazzon E, Crisafulli C, Di Paola R, Dal Bosco M and et al. Glycyrrhizin reduces secondary inflammatory process after spinal cord compression injury in mice. Shock 2009; 31:367-375.
- Chen G, Zhu L, Liu Y, Zhou Q, Chen H and Yang. Isoliquiritigenin, a flavonoid from licorice, plays a dual role in regulating gastrointestinal motility in vitro and in vivo. J Phytother Res 2009; 23:498-506.
- Nakanishi T, Inada A, Kambayashi K and Yoneda K. Flavonoid glycosides of the roots of Glycyrrhiza uralensis. Phytochem 1985; 24:339-341.
- Suresh Reddy Y, Sathyanarayana D and Kannan k. A Recent Phytochemical Review-Fruits of Tribulus terrestris Linn. J Pharm Sci Res 2016; 8:132-140.
- Jabbar A, Nazir A, Ansar NIlus, Javed F and Janjua KM. Effects of Terrestris tribulusto study on urine output and electrolytes in rabbits. Professional Med J 2005; 19:843-847.
- Neychev VK and Mitev VI. The aphrodisiac herb Tribulus terrestris does not influence the androgen production in young men. J Ethnopharmacol 2005; 101:319-323.
- Sharma. A Clinical Study on the Efficacy of Haridradi Kashaya in the Management of Madhumeha (Type2 Diabetes Mellitus), Int J Ayu Pharm Chem 2018; 9(1)eISSN 2350-0204.
- Abdullah N and Kasim KF. In-Vitro Antidiabetic Activity of Clinacanthus nutans Extracts. Int J Pharmacog Phytochem Res 2017; 9:846-852.
- Wickramaratne MN, Punchihewa JC and Wickramaratne DBM. In Vitro alpha amylase inhibitory activity of the leaf extract of Adenanthera pavonina. BMC Comple Alternat Med 2016; 16:466-471.
- Jong SK, Chong SKW and Kun HS. Inhibition of Alpha-glucosidase and Amylase by Luteolin, a Flavonoids. Biosci Biotechnol Biochem 2000; 64:2458-2461.
- Chunhe G, Han Z, Clarisa YP and Ken N. Evaluation of alpha-amylase and alpha-Glucosidase Inhibitory Activity of Flavonoids. Int J Food Nutri Sci 2015; 2:174-179.
- GawadeB and Farooqui M. Alphaâ??amylase Inhibitory Assay of Argemone Mexicana L. Leaves. Protein Sci 2017; 9:25-29.
- Gary DB, Yaoguang L and Stephen GW. The structure of human pancreatic alpha-amylase at 1.8 a resolution and comparisons with related enzymes. Protein Sci 1995; 4:1730-1742.

